zenterix
- 774
- 84
- TL;DR Summary
- I'd like to understand what a general and precise definition of an octahedral hole is when considering ionic close-pack structures.
I'm reading the book "Chemical Principles" by Atkins. In chapter 3H.6, entitled "Solids", there is a section that discusses a bonding model that explains structures and properties of many metals: the close-packed structure, in which spheres representing cations stack together with the least waste of space, "like oranges in a display".
We build up a close-packed structure in layers. Here are the first two layers:
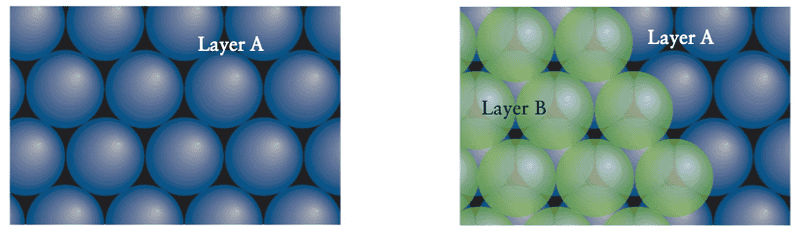
The third layer can be added in two different ways because there are two types of dips between the green spheres of the second layer: one type lies over the spheres of the first layer and the other type lies over the gaps in the first layer.
In the first case, the third layer duplicates the first layer and by adding successive layers we get an ABABAB... pattern called a hexagonal close-packed structure (hcp).
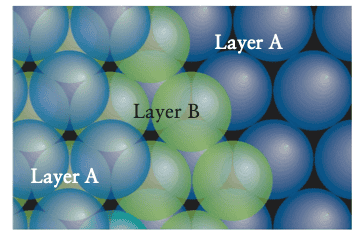
In the second case we end up with an ABCABCABC... pattern called a cubic close-packed structure (ccp).
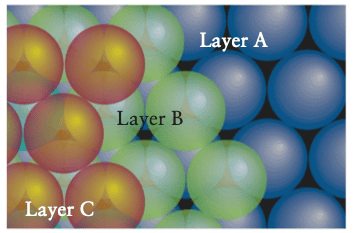
Then the text says that we can classify each type of dip as either a tetrahedral hole or an octahedral hole.
If a dip between three atoms is directly covered by another atom, the result is a tetrahedral hole. If a dip in a layer coincides with a dip in the next layer we have an octahedral hole, as we can see below
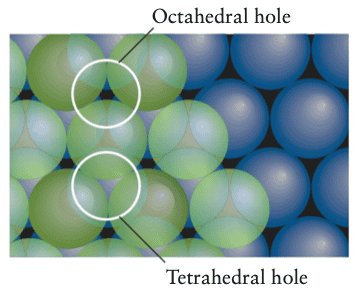
The tetrahedral hole is said to be formed by four atoms in the corners of a tetrahedron, and an octahedral hole is said to be formed by six atoms at the corners of an octahedron.
Now, the issue kind of starts with this last point. If we imagine a layer above the layer depicted above, then the octahedral hold would seemingly have nine atoms around it, since this third layer is no different from the first layer.
The issue for me really starts when we consider these holes in the context of ionic solids, e.g. sodium chloride.
Says the book,
Here is a depiction of the rock-salt structure
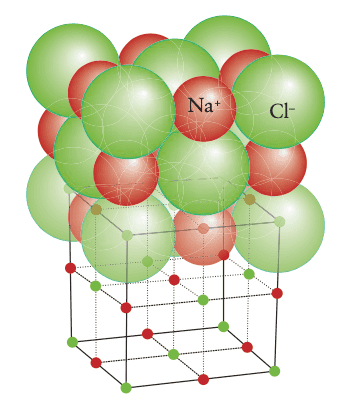
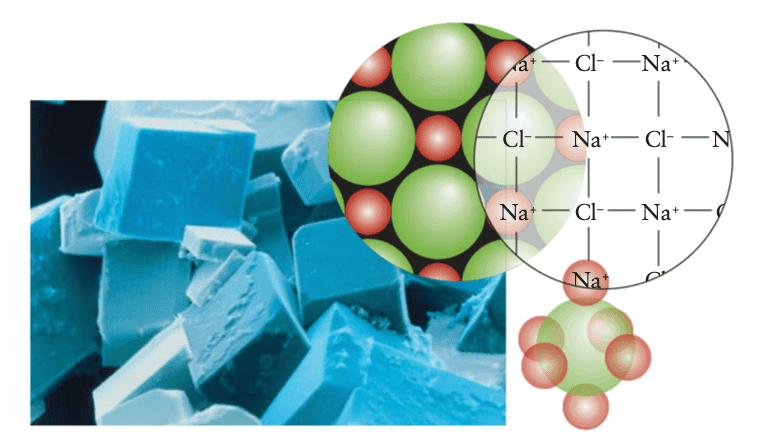
As far as the initial definition of an octahedral hole goes, I don't see any octahedral holes.
According to the initial definition, I would say that a hole in this structure is "tetrahedral" in the sense that there is an atom directly below any dip, but now it would be pentahedral.
After all, the initial definition counted atoms in the layer where the dip occurs, plus atoms in one other layer (either above or below). But now three layers are being counted.
So I am confused.
My question is: what is the exact general definition of an octahedral hole?
We build up a close-packed structure in layers. Here are the first two layers:
The third layer can be added in two different ways because there are two types of dips between the green spheres of the second layer: one type lies over the spheres of the first layer and the other type lies over the gaps in the first layer.
In the first case, the third layer duplicates the first layer and by adding successive layers we get an ABABAB... pattern called a hexagonal close-packed structure (hcp).
In the second case we end up with an ABCABCABC... pattern called a cubic close-packed structure (ccp).
Then the text says that we can classify each type of dip as either a tetrahedral hole or an octahedral hole.
If a dip between three atoms is directly covered by another atom, the result is a tetrahedral hole. If a dip in a layer coincides with a dip in the next layer we have an octahedral hole, as we can see below
The tetrahedral hole is said to be formed by four atoms in the corners of a tetrahedron, and an octahedral hole is said to be formed by six atoms at the corners of an octahedron.
Now, the issue kind of starts with this last point. If we imagine a layer above the layer depicted above, then the octahedral hold would seemingly have nine atoms around it, since this third layer is no different from the first layer.
The issue for me really starts when we consider these holes in the context of ionic solids, e.g. sodium chloride.
Says the book,
The rock-salt structure is a common ionic structure that takes its name from the mineral form of sodium chloride. In it, the ##Cl^-## ions lie at the corners and in the centers of the faces of a cube, forming a face-centered cube (FIG. 3H.28). This arrangement is like an expanded ccp arrangement: the expansion keeps the anions out of contact with one another, thereby reducing their repulsion, and opens up holes that are big enough to accommodate the ##Na^+## ions. These ions fit into the octahedral holes between the ##Cl^-## ions. There is one octahedral hole for each anion in the close-packed array, and so all the octahedral holes are occupied. If you look carefully at the structure, you can see that each anion is surrounded by six cations and each cation is surrounded by six anions.
Here is a depiction of the rock-salt structure
As far as the initial definition of an octahedral hole goes, I don't see any octahedral holes.
According to the initial definition, I would say that a hole in this structure is "tetrahedral" in the sense that there is an atom directly below any dip, but now it would be pentahedral.
After all, the initial definition counted atoms in the layer where the dip occurs, plus atoms in one other layer (either above or below). But now three layers are being counted.
So I am confused.
My question is: what is the exact general definition of an octahedral hole?