Container Definition and 334 Threads
-
S
I Container in an MRI room
So hypothetically you bring a container into an MRI room. Inside the container is a USB drive, a crypto hardware token, an electronic tablet, and a Walkman. Then you run the room's MRI machine, which has a magnetic field strength of 3 Teslas. What kind of container could prevent any...- Sodagit
- Thread
- Container Magnetic Shielding
- Replies: 12
- Forum: Other Physics Topics
-

Measurements Calculation Problem
I have done this problem three times now, and keep coming up with an answer that is a factor of 10 off from the answer in the solutions manual. The way I'm doing it is MUCH different than how they did it, so I can't use their method to see where I'm going wrong. I want to find out what I'm...- Ascendant0
- Thread
- Calculation Container Measurement
- Replies: 3
- Forum: Introductory Physics Homework Help
-
A
Waste Container Handling Crane -- Why are we getting this lift offset?
I am dealing with a waste container handling crane. Waste container is 12000kg. It is lifted by a grapple by twist locks at each corner. Height of lift is 14m. The contents of the container leads to an offset c of g which cannot be avoided. The containers are to be stacked 6 high. The crane...- AlanThomas
- Thread
- Container Crane
- Replies: 13
- Forum: General Engineering
-
M
Change in reading of the scale when putting ball in a container of alcohol
The container and water inside it are at rest. ##F_{net,y}=ma_y## ##N - (m_{container}+m_{water})g = 0 ## ##N = (m_{container}+m_{water})g## ##m_{container}+m_{water}=700g## ##m_{container}+\rho_{water} V=700g## ##m_{container}+0.8(10*10*8)=700g## ##m_{container}=60g=0.06kg## Now we put a ball...- MatinSAR
- Thread
- Container homework help Physics
- Replies: 18
- Forum: Introductory Physics Homework Help
-

B Cooling liquid in rigid container
Hello, I have a sealed and rigid container, (can't change volume, can allow heat through), 1.0m x 1.0m x 1.0m in size, that is completely full with warm water, let's say at 40°C. The initial pressure in the container is 1atm and the volume of the water at 40°C perfectly matches the volume of the...- ABC83
- Thread
- Container Cooling Liquid
- Replies: 25
- Forum: Thermodynamics
-
R
Hydrostatic pressure in a narrow container
I understand that pressure increases with depth regardless of the shape of the container. However, this doesn't sit well with me. Imagine a container: 8' tall x 1" wide x 1' long. The pressure at the bottom of the container is 8' x 62.4 pcf = 499.2psf. However, the weight of the water in the...- RFreund
- Thread
- Container Hydrostatic Hydrostatic pressure Pressure
- Replies: 16
- Forum: General Engineering
-
V
B Collision time interval of a gas molecule with wall of container
I have been trying to make sense of the derivation of pressure under Kinetic Theory of Gases chapter, but it's not making sense to me when the impulse momentum equation is used for the collision between a gas molecule and the wall of the container. The book says that for the elastic collision...- vcsharp2003
- Thread
- Collision Container Gas Ideal gases Impulse momentum Interval Kinetic theory Molecule Time Time interval Wall
- Replies: 6
- Forum: Thermodynamics
-
C
Pressure in a gas container measured with a barometer and a U pipe
Can someone please tell me where I am wrong, here goes the question: to a container filled with gas, U shaped pipe is attached, as shown in the picture(picture below). What is a gas pressure in the container if the height of the pillar of mercury in barometer is 740 mm? The way I solved it is...- Callmelucky
- Thread
- Atmospheric pressure Container Gas Pipe Pressure
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Time to evaporate LN2 from a container
Hello, Could someone please help me understand how to approximate how long it will take for liquid nitrogen in a room temperature container to completely evaporate. Here's the scenario: I have a metal container (41x13x15") filled with 5.5" of liquid nitrogen (just released from a Dewar ~ 320F)...- pve
- Thread
- Boiling Container Liquid nitrogen Thermodynamics Time
- Replies: 8
- Forum: Materials and Chemical Engineering
-
A
I Fluid flow through a pin-hole of x diameter in a closed container
Greetings, I've come across lots of exercises regarding Bernoulli's equation. However, never seen one where the top of the vessel is closed, and fluid flow exists via gas (air) going in. Has this problem been studied in the past? Assume a cylindrical vessel filled to the maximum with a D-sized...- AspiringMonkey
- Thread
- Closed Container Diameter Flow Fluid Fluid dynamics Fluid flow
- Replies: 2
- Forum: Mechanics
-
C
How to neutralize bromine that's eaten through it's container?
I'm not sure exactly how this happened as it was before I was employed here more than half a year ago, but I believe bromine liquid was stored improperly in one of our chemical cabinets. It ate through the container and saturated a small portion of the bottom of the cabinet. The spill was... -
B
B Does gas expanding in a container cause displacement of the container?
If particles are trapped in one half of a massless container by a barrier, and the barrier is removed so that the particles are allowed to expand to fill the whole container, it seems the center of mass would be displaced to the center of the container, whereas before it was located at the...- bobdavis
- Thread
- Cause Container Displacement Gas
- Replies: 11
- Forum: Classical Physics
-
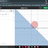
Pressure due to a Liquid on container Wall
I have just started this topic so i Don't have much clearity about it . In our school it was tought that Pavg = (Pi + Pf)/2 ...(i) If we have a cuboidal shape container then it should be pgh/2 ryt ? But also Pavg = Ftotal on the wall /A ...(ii) =...- SpectraPhy09
- Thread
- Container Liquid Pressure Wall
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Two immiscible liquids in a container
I really need a help with this exercise: A ##1.75##-m-high container has two immiscible liquids stacked on top of each other. The upper liquid has specific gravity ##SG = 0.45## and the other has density ##\rho = 61.78\, lbf/ft^3##. If the pressure exerted by the lower liquid at the bottom is...- luciriv
- Thread
- Container Liquids Pressure calculations
- Replies: 6
- Forum: Engineering and Comp Sci Homework Help
-
P
Does the colour of a container affect how fast water in it cools?
Containers are painted with the 7 colours of the rainbow; each colour corresponds to a different wavelength. Pour boiling water into each container. Which container would cool the fastest? Does the result change depending on whether the exterior or the interior or both are painted? The material...- phantomvommand
- Thread
- Colour Container Water
- Replies: 22
- Forum: Mechanics
-
A
Force on two cables holding a liquid-filled container against a wall
Before, sorry for my English, it is not my native language I already have the solution to the issue, I just didn't understand a step. 1) Calculation of weight force (vertical): $$ F_v = \frac{\rho \pi R^2 . L .g}{4}$$ 2) Calculation of force due to water pressure on the plate (horizontal)...- A13235378
- Thread
- Cables Container Force Wall
- Replies: 2
- Forum: Introductory Physics Homework Help
-
S
Question about water container with hole at the bottom
(a) $$V=\int_{0}^{x} A~dx$$ $$=\int_{0}^{x} f(u) dx , \text{u is dummy variable}$$ Is this the answer? Or there is something else I can do to continue the working? (b) $$\frac{dV}{dt}=\frac{d}{dt} \int_{0}^{x} f(u) dx=f(x).\frac{dx}{dt}$$ Is this correct? (c) $$\text{time}=\frac{\text{rate...- songoku
- Thread
- Container Hole Water
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-
K
Iron block dropped into a container of water sitting on a scale
Does the scale change during the block sinks? I am so confused... Is there any difference on change of scale with the applying of drag force?- Kate_12
- Thread
- Block Buoyancy force Container Drag force Iron Scale Water
- Replies: 12
- Forum: Introductory Physics Homework Help
-
S
Riemann Sum to find the time to fill a container
(a) I imagine there are several rectangles to represent the area under graph of p vs t then I try to make equation for the total area. Since the question asks about time when the container holds 22 fewer liters than it does at time t = 9, I think the total area of rectangles starting from t = b...- songoku
- Thread
- Container Riemann Riemann sum Sum Time
- Replies: 12
- Forum: Calculus and Beyond Homework Help
-

Chemistry Pressure of gasoline in a closed container
If I knew how much of the gasoline evaporated, then I could use p = nRT/V to fine the partial pressure of the gasoline and add it to the pressure of the atmosphere. So my question is, How do I find how much of the gasoline will evaporate. I would think that the gasoline would evaporate until it...- barryj
- Thread
- Closed Container Gasoline Pressure
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Freeing the stuck container ship Ever Given in the Suez Canal
[Mentor Note -- Interesting engineering discussion from the Weird News GD thread moved to the General Engineering forum as its own thread] How That Massive Ship Got Stuck in the Suez Canal—and Why Nobody Can Get It Out...- Astronuc
- Thread
- Container Ship Stuck
- Replies: 72
- Forum: General Engineering
-
F
Emptying a container and Efflux Speed
Hello Forum, The speed of efflux ##v_{efflux}## of a liquid, say water, from an orifice in the lower part of a container depends on the vertical distance between the free surface of the fluid and the lower position of the orifice in the container itself. The faster the speed ##v_{efflux}## the...- fog37
- Thread
- Container Speed
- Replies: 6
- Forum: Mechanical Engineering
-

Cylindrical container with a piston
Please help by answering ALL parts- mavidias
- Thread
- Container Cylindrical Piston
- Replies: 2
- Forum: Introductory Physics Homework Help
-
S
What time is needed to move water from a pool to a container?
I take position 1 as the surface of the pool and position 2 as the surface of the container so the value of ##P_1 = P_2 = P_{atm}## and ##v_1=0## and ##h_2=0## ##P_1 + \rho gh_1 + \frac{1}{2} \rho {v_1}^2 = P_2 + \rho gh_2 + \frac{1}{2} \rho {v_2}^2## ##\rho gH = \frac{1}{2} \rho {v_2}^2##...- songoku
- Thread
- Container Time Water
- Replies: 47
- Forum: Introductory Physics Homework Help
-

Pressure at the walls of a container
I wrote energy of each molecule is ##\frac{1}{2}mv_{rms}^2 \Rightarrow E/3 = \frac{1}{2}mv_i^2##. So total energy of each type of gas is ##E_i = N_i\frac{3}{2}mv_i^2## Now, PV=E. So total Pressure = Sum of energies/volume ##P=\frac{\frac{3m}{2}\sum_i N_i v_i^2}{V} =\frac{3m}{2} \sum_i n_i...- Kaguro
- Thread
- Container Pressure
- Replies: 1
- Forum: Introductory Physics Homework Help
-
U
Build a pressurized container to infuse herbs
First off I would like to thank anyone and everyone that reads and or contributes any guidance to my problem. I want to build a pressurized container that can hold 10lbs of an herb, ex. mint , so I can infuse it with a flavor, ex. vanilla. I have the infusion process worked out. I just need...- uscmedina
- Thread
- Build Container
- Replies: 17
- Forum: Mechanical Engineering
-
C
Maintaining higher air pressure in an "open" leaky container
Hey all, I've got a container that needs to maintain a certain amount of air pressure for the contents inside to develop correctly. However, it is an open container (like a big box with a hole in it), so without a pump constantly pushing air into it, it will rapidly drop pressure to equalize...- cashflow
- Thread
- Air Air pressure Container Pressure
- Replies: 20
- Forum: General Engineering
-

Locking gear on container doors
Hi there, I would like to use Your expertise in solving one of the challenges I've faced right now, as an intern. My task is to design steel container doors and its locking system. As it's a light container, locking system should be placed inside of the doors. To visualize, the container and...- Optymista93
- Thread
- Container Gear
- Replies: 3
- Forum: Mechanical Engineering
-

Ideal gas in a cylindrical container
It looks more like a computational obstacle, but here we go. Plugging all of these to the partition function: $$Q = \frac{1}{N! h^{3N}} \int -\exp(\frac{1}{2m}(p^2_{r}+p^2_{\phi}/r^2+p^2_{z})+gz)d\Gamma=$$ $$= \frac{1}{N! h^{3N}} \int \exp{(\frac{-1}{2m}p^2_{r})}dp_{r_{1}}...dp_{r_{N}}...- CptXray
- Thread
- Container Cylindrical Gas Ideal gas Statistical mechanics
- Replies: 4
- Forum: Advanced Physics Homework Help
-
E
Internal energy of a container filled with water & steam
I've worked out that half of the steam must condense (since the pressure of the steam needs to remain constant for the forces on the massless piston to balance). Also, if the total volume of the container is ##V##, the work done on the system equals ##\frac{P_{atm}V}{4}##. When half of the steam...- etotheipi
- Thread
- Container Energy Internal Internal energy Steam Water
- Replies: 12
- Forum: Introductory Physics Homework Help
-
F
Moving an adiabatic partition in an adiabatic container
The actual data for the problem and my (and my friend's) attempt at a solution are in the attached file. In a nutshell, this is what happened. I obtained a solution based on the fact that the system is isolated. Thus the initially hot gas moves the partition doing work onto the initially cold...- FranzDiCoccio
- Thread
- Adiabatic Adiabatic process Container Ideal gas Partition
- Replies: 49
- Forum: Introductory Physics Homework Help
-
G
MHB Finding the Minimum Number of White Balls in a Container with 27 Balls
We have 27 balls in the container, some of which are white and some black. How many white balls in the container must be at least, so that the probability that two black balls were drawn at random without a return was less than 23/30?- ghostfirefox
- Thread
- Balls Container Minimum
- Replies: 2
- Forum: General Math
-
P
B How Can Photons Contribute Mass to a Container?
My question comes from reading the wikipedia page on mass-energy equivalence. The statement would seem to be contradictory: So photons contribute to the energy (and therefore mass) of the container; but photons are massless - that is, they have 0 rest mass. But doesn't this mean that we're...- PhyCurious
- Thread
- Container Mass Photons
- Replies: 16
- Forum: Special and General Relativity
-

Effect on air pressure and volume in an enclosed container
Summary: I wish to understand if bubble formation in milk while being sloshed around, or the formation of separation layers will affect both the pressure and volume of the air head space above the milk Hi all : ) I have a basic physics question and sorry if its a very silly question: Let's...- falcon999
- Thread
- Air Air pressure Container Pressure Volume
- Replies: 2
- Forum: General Engineering
-

Why doesn't pressure depend on the shape of the container?
As we know that liquids(same) at same height exert same pressure because of height difference but as I have a question in which there are some figures In which the (d) part lower part is extended horizontally,so by common sense the pressure in horizontal direction should increase But why at... -

Pendulum with a mass on a container (thermodynamics problem)
The kinetic energy of the pendulum ##K=\frac{1}{2}\cdot m\cdot v^2## will turn into heat (entirely). So both the air and the block of iron will change their temperature. To find ##n## (moles of the gas) I can use the ideal gas law: ##n=\frac{pV}{RT}=0.9 mol## Do I have the following equation...- ValeForce46
- Thread
- Container Mass Pendulum
- Replies: 10
- Forum: Introductory Physics Homework Help
-
K
Is it safe to use 75% Alcohol with PP and other plastic-made container
Is it safe to use 75% Alcohol (Isopropyl) with PP and other plastic made container commonly found in the consumer market for cleaning? Could there be any reaction and release toxic / harmful gases or materials? I once studied Chemistry but left school for decades -

What are the forces acting on the container?
Let’s say I bring a closed container full of air into deep space where atmospheric pressure is is near 0. I Open the lid and then the air moves out of the container and into the vacuum of space. What are the forces on the container AFTER the lid has been removed? Does the container move...- Chuzzled
- Thread
- Container Forces
- Replies: 42
- Forum: General Engineering
-

Heat Transfer of a Dewar Nitrogen Container
Hello guys, :smile: So like the Titels says I am Trying to get an Approximation of the Heat Transfer which ocours in a Deware container. Unfortunately all my calculations so far seem to suggest way to low Boil off rates for my Container its just to efficient, here is a summary of my Attempts...- rikku95
- Thread
- Container Heat Heat transfer Nitrogen
- Replies: 1
- Forum: Materials and Chemical Engineering
-
M
Misc. Where can I purchase this Acrylic container?
Didn't know where to put this, but does anyone know where to purchase a rectangular acrylic box (top open, so I can fill with liquid) with dimensions 3" high by 4" long by 2" wide? I appreciate any suggestions you have!- member 428835
- Thread
- Container
- Replies: 13
- Forum: DIY Projects
-
C
Can gas kinetic theory explain heat transfer from gas to a container?
If one considers the kinetic theory of gases, can a first order estimate of thermal transfer be performed by considering momentum exchange at the container's surface? I understand the basics of explaining and calculating pressure with the kinetic theory of gases, but if we assume energy is...- cmb
- Thread
- Container Explain Gas Heat Heat transfer Kinetic Kinetic theory Theory
- Replies: 6
- Forum: Thermodynamics
-
D
Can Light Oil Be Used as a Transfer Agent in an Ultrasonic Cleaner?
Want to do same but in glass container instead of directly in the reservoir as he did. Called the manufacturer, got transferred to their "lab guy" who seemed bumfuzzled by what I want to do, dis-recommended plain water in the reservoir, citing potential corrosion issues??, recommending their...- Dwayne Oxford
- Thread
- Container Ultrasonic
- Replies: 1
- Forum: General Discussion
-

Hole Sizing to Drain Fluids [Pressurized Container]
Hello, I want to size my system to be able to get rid of fluids without any head buildup within the container. I am just a bit confused as to what formula I should use. My problem is summed up in the following schematic. Note that P1>P2, I have assumed H=10^(-4)m and my flow rate is 0.1 m3/s...- nn2e19
- Thread
- Bernoulli Container Fluid Fluids Hole Pipe flow Sizing
- Replies: 1
- Forum: General Engineering
-
B
Calculating Displacement of X and Y with Water-Filled Container
NO TEMPLATE BECAUSE ORIGIONALLY SUBMIIED TO NON-homework forumHi, I have a pipe connects to a container's outlet. The container contains water and the pipe is open-ended. When a 10Kg object is placed inside the container and compress the water. Can you tell me how to calculate the displacement...- bbd001
- Thread
- Container Displacement
- Replies: 7
- Forum: Introductory Physics Homework Help
-

Pressurized gas container gets opened
Homework Statement I have an empty 2-Liter bottle. It contains 3 g of air inside with an initial air pressure of 1105 mb. When I open it (which is an adiabatic process), I release the pressure which is instantaneous. The pressure then becomes standard atmospheric pressure. What is the...- dkeating
- Thread
- Calculus Container Gas Physics Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Shape of a bubble inside a rotating container
1. A closed cylindrical vessel filled with water (at room temperature) contains a small air bubble of normal pressure and volume ##V=1~{cm}^3## inside in it.The cylinder is then started to be rotated slowly with a small angular acceleration in a complete weightlessness (at a space station)...- Raihan amin
- Thread
- Bubble Container Fluid dynamics Rotating Shape Surface tension Thermodaynamics
- Replies: 5
- Forum: Advanced Physics Homework Help
-
L
Pressure while heated - half full or full container
Hi I was in a discussion with some friends a couple of days ago. The discussion revolved the pressure raise in a liquid filled container. The question was, is the pressure behavior dependent on whether the container is filled with only water or filled 50/50 water/air. (The discussion started...- liquidFuzz
- Thread
- Container Pressure
- Replies: 9
- Forum: General Engineering
-

Pressure at the bottom of a container containing two liquids
Homework Statement (In the attachment)There is two layers of liquids in the container .The density of the upper layer of the container, ##ρ_1=800kg/m^3## and the density of the bottom layer of the container ,##ρ_2=13600kg/m^3## .What is the pressure at the bottom of the container? Homework...- Akash47
- Thread
- Container Fluid Liquids Pressure
- Replies: 6
- Forum: Introductory Physics Homework Help
-
S
Confused about the volume of a rigid (glass) container
Homework Statement This is for a lab report and I am just confused about how I should regard volume because this issue is affecting 3 other questions. If you heat a gas in a closed flask that is connected to a manometer with water in it, the water level goes up (the arm open to atmosphere rises...- sp3sp2sp
- Thread
- Confused Container Glass Volume
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
How is DWT in container ships achived?
Hi there, in martime terms DWT includes the containers weight as well. What i don't understand is how is it achieved? All ships specifications of container capacty has higher weight than the DWT.- karamss
- Thread
- Container Ships
- Replies: 10
- Forum: General Engineering