You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Entropy Definition and 1000 Threads
-
A
Ball does not bounce automatically because of entropy?
When reading about entropy it states that ball does not automatically bounce up because of entropy. It is just not favorable. I don't get this. Isn't the correct reason gravity? Ball does not bounce up because of gravity! Where does entropy come from?- Avichal
- Thread
- Replies: 10
- Forum: Thermodynamics
-

Can the Entropy of an Isolated System Decrease?
So in my physics textbook, the 2nd law of thermodynamics stated in terms of entropy reads "the entropy of a closed system can never decrease." Now, shouldn't it indicate the entropy of an isolated system can never decrease. All other sources I've looked at note an isolated system, as well...- NATURE.M
- Thread
-
- Tags
- Closed Closed system Entropy System
- Replies: 7
- Forum: Thermodynamics
-
N
Entropy: Increasing or Decreasing?
Everybody says entropy of the universe is increasing. But after the big bang, matter formed, it came together, stars and galaxies were formed, on a small planet called Earth atoms/molecules came together to form unicellular organisms and then higher life. Our brains are organizing even more...- Naveen3456
- Thread
-
- Tags
- Entropy
- Replies: 4
- Forum: Thermodynamics
-
F
Maximum Entropy in Gaussian Setting
Hello, I have a doubt about the distribution of random variables that maximize the differential entropy in a set of inequalities. It is well known that the Normal distribution maximizes the differential entropy. I have the following set of inequalities: T1 < I(V;Y1|U) T2 < I(U;Y2) T3 <...- ferreat
- Thread
- Replies: 2
- Forum: Set Theory, Logic, Probability, Statistics
-
A
Why is there zero resistivity in superconductors when there is non-zero entropy?
According to theory of superconductivity, resistivity almost zero. Below critical temperature the entropy decreases markedly with cooling . why resistivity zero when entropy not equal to zero? my doubt is when there is an entropy there is a disorder. then how can move conducting electron...- anuraj.b
- Thread
-
- Tags
- Entropy Superconductivity
- Replies: 4
- Forum: Atomic and Condensed Matter
-
B
A Tricky Problem Involving Entropy
So we learn in basic thermo that for any system, the derivative of entropy with respect to volume is pressure over temperature: ∂S/∂V=P/T. Suppose we have a box with two partitions, and a moveable wall in between the partitions. The box and wall are both insulated to prevent heat transfer...- BucketOfFish
- Thread
-
- Tags
- Entropy
- Replies: 4
- Forum: Thermodynamics
-
M
Entropy in Cosmology: Exploring the Universe
So we know that at the moment of the big bang the universe was relatively low in entropy; as the universe has expanded entropy has increased, but what about further down the line? What about when the universe is so cold that it's nothing more than a collection of black holes, and further more...- m_robertson
- Thread
- Replies: 7
- Forum: Cosmology
-
S
Adiabatic expansion, entropy change
Homework Statement 1 mol of monoatomic ideal gas (temperature T1) is inside a cylinder with a moving piston (all are isolated). The initial external pressure on the piston is P1. at some point the external pressure is changed to (2/3)P1, the gas undergoes (irreversible) adiabatic expansion...- susdu
- Thread
- Replies: 12
- Forum: Advanced Physics Homework Help
-

Significance for LQG of Sen's result on entropy of black holes?
Sen 2013 says, How serious a problem is this for LQG? Does this mean that LQG doesn't have GR as its semiclassical limit? Does that mean it's a dead theory, or maybe just that it needs to be modified? Is the technique using Euclidean gravity reliable? Since I'm not a specialist, I'd be...- bcrowell
- Thread
- Replies: 8
- Forum: Beyond the Standard Models
-
F
Why is Cpln(T2/T1) Zero for Isothermal Entropy Change?
Can someone explain to me why Cpln(T2/T1) = 0? Thank you.- freshbox
- Thread
-
- Tags
- Change Entropy Isothermal
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
How Do You Calculate the Change in Entropy When Ice Melts in Water?
In 250gr water at 23 oC(degrees celsius) we throw in 27gr ice at 0 oC. Find the change in entropy. (sorry if that was a bad translation but English is not my native language) The answer is 0.78 calories/oC. But I'm not sure how do this. The formula I have for entropy is: S=δQ/T I find the...- devil0150
- Thread
- Replies: 1
- Forum: Thermodynamics
-
E
Entropy increase,coarse-grained vs. Landauer's principle justification
First off, just clarify that I have a very, very superficial knowledge of Physics, so my apologies if my question is based on an obvious misunderstanding of the basic principles underlying the second law of thermodynamics or if it has a rather simple answer. The doubt that I have is related...- Evaristo
- Thread
- Replies: 4
- Forum: Thermodynamics
-

Loop Quantum Gravity and entropy
How specifically does LQG explain how the universe can oscillate from big bang to big crunch ad infinitum? Wouldn't the total energy able to be used as work decrease after a couple of bounces? Am I simply misunderstanding or making a false assumption about what LQG's premises are? I've not...- Digitalism
- Thread
- Replies: 8
- Forum: Beyond the Standard Models
-
L
How Is Entropy Calculated for a Density Matrix with Eigenvalues 0 and 1?
Homework Statement Calculate entropy for density matrix with eigenvalues ##0## and ##1##. Homework Equations ##S=-\lambda_1 \ln \lambda_1-\lambda_2 \ln \lambda_2## where ##\lambda_1## and ##\lambda_2## are eigenvalues of density matrix. The Attempt at a Solution How to calculate...- LagrangeEuler
- Thread
- Replies: 8
- Forum: Advanced Physics Homework Help
-
V
Relating physics forces and entropy
Consider proton and electron are separated in space at some finite distance.Now suppose they released then the decrease in potential energy equals kinetic energy but as electrons or protons are accelerated they emit em and lose some gained kinetic energy.Also the mass is another form of...- vishvajeet
- Thread
- Replies: 2
- Forum: Thermodynamics
-
T
Entropy and electromagnetic radiation
I don't understand this: According to what modern physicists believe to be true, there is entropy that slowly converts all energy of the universe into heat that cannot do any work. Than this heat is radiated as infrared light into space. Correct? Besides infrared heat radiation, start also...- tarakan
- Thread
- Replies: 4
- Forum: Electromagnetism
-
S
Entropy of a rubber-band (force determined by entropy or energy)?
Homework Statement The resultant of a experimental run of a force (N) vs temperature (K) gives me a slope of 0.0039x - 1.0929. The slope has a (∂f/∂T)l = (∂S/∂l)T Homework Equations Equation (11) attached as PNG file We can obtain all the quantities of the right side of Eq. (11)...- sdnnet3
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
S
How meaurements increase entropy?
¿ How meaurements increase entropy? The decoherence before the measurement increase entropy but collapse returns the state of the system to a pure state. ¿Why then measurements increase entropy- StarsRuler
- Thread
- Replies: 9
- Forum: Quantum Physics
-
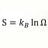
Entropy Of Mixing and Specific Heat
Homework Statement 100g of lead, specific heat .0345 cal/g/C at 100 C is mixed with 200 g of water at 20 C. Find the difference in entropy of system at end from value before mixing. Note: I think the book has wrong answer of 1.42 cal/K Homework Equations Before mixing entropy for water =...- morrobay
- Thread
- Replies: 17
- Forum: Introductory Physics Homework Help
-
G
Will the fusion reactor ITER decrease entropy?
ITER is the fusion reactor in southern France that hopefully will come online in 2018. According to wiki http://en.wikipedia.org/wiki/ITER The ITER fusion reactor itself has been designed to produce 500 megawatts of output power for 50 megawatts of input power. Does this mean that if...- g.lemaitre
- Thread
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
B
Which has least entropy- living organism or same mass as chrystal?
Which has the least entropy? A 1kg diamond vs 1kg living organism I've gather that living things are generally at a state of low entropy compared to the same matter in other configurations- as though complexity goes hand in hand with low entropy. Yet a perfect chrystal is supposedly low...- bcrelling
- Thread
- Replies: 11
- Forum: Thermodynamics
-
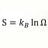
Entropy For Irrevesible Process
Homework Statement Two 100g Aluminum cubes with T1 = 80° C and T2 = 20°C Specific heat of Al is .22 cal/g/°C. The cubes are placed in contact and in short period of time a quantity of heat, dQ , is transferred from T1 cube to T2 cube. The cubes temperatures now are 75°C and 25°C. After a...- morrobay
- Thread
- Replies: 9
- Forum: Introductory Physics Homework Help
-
G
Entropy problem involving saltwater uptake into a cell
Homework Statement For public health reasons, you are investigating small systems that turn sea water into drinking water. One portable system takes a volume of saltwater and produces two thirds that volume of freshwater with an increased concentration of salt in the other third of the volume...- GoGoGadget
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
A
Why is entropy a driving force for a reaction
I understand the Gibbs Free Energy equation that was drilled into us in Chemistry class but not even my teacher could explain what entropy had to do with free energy, and furthermore, why entropy is a driving force for a reaction. From what I understand, entropy is the distribution of energy...- arp001
- Thread
- Replies: 11
- Forum: Thermodynamics
-
B
Move A->B What about entropy change?
move A-->B ...What about entropy change? Hi, I have a physical question regarding entropy, temperature, internal energy and mechanical energy. The situation is following: Two objects are located inside a one dimensional frictionless and adiabatic space where object “a” is an observer that...- bolly
- Thread
- Replies: 25
- Forum: Thermodynamics
-
D
Can a Black Hole's High Entropy State Coexist with Supercondensed Matter?
How can a black hole have a high entropy state if the matter inside it is in a supercondensed form with maximum density,which means less degrees of freedom therefore less entropy than normal matter.Like in neutron stars we have low entropy with the neutron degenerate matter and yet a black hole...- Double-Slit
- Thread
-
- Tags
- Black hole Entropy Hole
- Replies: 14
- Forum: Cosmology
-
K
Entropy change during wet compression
I have a quick question. Consider the process S2 to S1 in the figure below Since this represents wet (isentropic) compression, mathematically we have ΔS=0 (assuming adiabatic compression). But if we consider the process in a physical way, we are going from a region of less disorder...- kulkajinkya
- Thread
-
- Tags
- Change Compression Entropy
- Replies: 6
- Forum: Thermodynamics
-
K
What is the Shannon Entropy of the String 'QXZ'?
Shannon entropy of "QXZ" Hello everyone. I am trying to determine the Shannon entropy of the string of letters QXZ, taking into consideration those letters' frequency in English. I am using the formula: H(P) = –Ʃ pilog2pi What's puzzling me is that I am expecting to calculate a high...- Karl Coryat
- Thread
-
- Tags
- Entropy Shannon entropy
- Replies: 2
- Forum: Set Theory, Logic, Probability, Statistics
-
C
Deriving Peng-Robinson Entropy Departure Function
EDIT: Nevermind. I figured it out. The two expressions are, in fact, equal. An excerpt from a book at this link, http://webpages.sdsmt.edu/~ddixon/Departure_Fxns.pdf, states that the entropy departure function for any equation of state is equal to the following (Eqn. 4.4-28)...- cjc0117
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
B
Does entropy drive our conscious experience of time?
From what I can gather, the laws of physics are time sysmmetrical- the exception being the second law of thermodynamics. So could it be that our whole experience of time is driven souly by increasing entropy? Assuming that we live in a deterministic universe(controversial I know) all past...- bcrelling
- Thread
-
- Tags
- Drive Entropy Experience Time
- Replies: 5
- Forum: Thermodynamics
-
H
Exploring Entropy: Order vs Disorder in Physical Systems
In my TD classes I have to deal with entropy. But what does it really mean? I know that it is the degree of disorder and the equation S=q/t. But what is this disorder? How can a physical system be aware of order and disorder since it is an abstract concept?They say that solids have high order... -
C
Is the Universe Organized or Chaotic in its Younger Years?
I was thinking maybe the universe isn't in the chaotic state its meant be in. Rather a more organised state and the universe is still in it's younger years. This is just a thought any insight would be great.- capcom1983
- Thread
- Replies: 11
- Forum: Cosmology
-
D
Entropy change when converting water to steam
Homework Statement At constant atmospheric pressure, 20g of water at 30C is converted into steam at 250C. Assume the heat capacity of liquid water is constant at 4.2 J/gK and the heat of vaporization at 100C is 2260 J/g. The molar heat capacity of water vapor at constant pressure is given by...- doombanana
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
W
Entropy change in isochoric process
Homework Statement Hi everyone In an isochoric process ( a solid heat body with T1 temp. connected with heat bath with T2 temp) we could calculate its entropy change(heat body) from dS = Cv/T dT and entropy change of heat bath from dS = dQ / T2, using dQ = Cv dT in 1st equation ( i...- wowowo2006
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
Entropy Change of a Monatomic Ideal Gas Compressed Adiabatically
Homework Statement A monatomic ideal gas is compressed adiabatically. What is ΔS of the gas? n = 1.00 vi = 10.00 L vf = 4.00 L Constant External Pressure = 50.00 atm Homework Equations ΔU = q + w = 0 + (-PextΔV) ΔS = ∫[(qrev)/T]dQ The Attempt at a Solution In order to...- Basis
- Thread
-
- Tags
- Change Compressed Entropy Gas Ideal gas
- Replies: 5
- Forum: Introductory Physics Homework Help
-

The Entropy of Information Source
In the paper "A Mathematical theory of Communication" by Shannon I couldn't understand a theorem (Theorem 4) under the topic "The Entropy of Information Source"- Here 'H' is the entropy. Can you explain me how we get this theorem? What is this n(q)? I couldn't understand the sentence...- iVenky
- Thread
-
- Tags
- Entropy Information Source
- Replies: 4
- Forum: Electrical Engineering
-
A
Boltzmann's entropy/ Microcanonical Entropy
Hey guys, i am reading something about entropy. :confused: And got a question. The Boltzmann entropy is defined by: S=k\cdot \ln{W} W is the number of microstates connected to an given macrostate. The entropy of the microcanonical ensemble(fixed given Energy) is defined by...- Abigale
- Thread
-
- Tags
- Entropy
- Replies: 1
- Forum: Thermodynamics
-
W
Entropy Generation of an Electric Motor: Analyzing du/dt & dS/dt
For the following question, the solution manual sets du/dt (change of internal energy) = 0. Moreover, it also set the change of total entropy to zero. Why is that? An electric motor under steady load draws 9.7 amperes at 110 volts; it delivers 0.93 kW of mechanical energy. The temperature of...- wynnl
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

A Gravitational Entropy proposal from Ellis, Tavakol, and Clifton
It's interesting that the entropy of the gravitational field (i.e. the entropy of geometry) has never been satisfactorily defined. Since geometry is such an important part of the picture near the start of expansion, this means that total entropy around then has been undefinable, leaving...- marcus
- Thread
-
- Tags
- Entropy Gravitational
- Replies: 1
- Forum: Cosmology
-
G
Entropy Properties: Correct & Incorrect Expressions
hello, i have been given two formulae for the entropy of an ideal gas undergoing a reversible process. the correct expression is: S = Nk(s0-ln({\frac{N_0}{N}})^{5/2} + ln({\frac{T}{T_0}})^{3/2} + ln({\frac{V}{V_0}})) where s_0 is a constant, N the number of particles, T the...- Guffie
- Thread
-
- Tags
- Entropy Properties
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Quantum physics perspective on radiation entropy
Hi, I've been curious about his subject for quite a while now, and I figure I need to ask here in these forums to get started in the right direction in understanding this. In classical thermodynamics, we define entropy: ds = \delta q / T However, when it comes to radiation in quantum...- jlefevre76
- Thread
- Replies: 1
- Forum: Quantum Physics
-

Entropy Variation - System, Neighborhood and Universe
I learned ΔSuniv = ΔSsys + ΔSneib If ΔSuniv > 0 the process is spontaneous. But ΔS = Q/T right? Shouldn't Qsys = -Qneib? (The heat released by the system = - heat released by the neighborhood) I cannot understand why this is wrong! If it were the case, ΔSuniv would be always zero... -
C
The photon, time and entropy
The photon, "time" and entropy (ignore original title, in fact id appreciate if a mod would change it. It was not my intention to have a conversation about time, lol!) Can we view a closed system of just photons, as ever, or usually, being subject to entropy (defined by the whole 2nd law...- Curious45
- Thread
- Replies: 16
- Forum: Quantum Physics
-
S
Exploring the Connection between Phase Space and Entropy in Particle Decay
Is there any relationship between phase space and entropy? regarding to decay of particles- shakeel
- Thread
-
- Tags
- Entropy Phase Phase space Space
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
T
What is the role of Shot and Scold in the entropy equation?
Homework Statement In the entropy equation 1S2 + ∫δQ/T = S2 - S1, what is the difference between 1S2 and ∫δQ/T? So for example if we have two reservoirs, one hot and one cold and heat is transferred in between we have: Shot = Q/Thot Scold = Q/Tcold Where do the Shot and Scold terms...- theBEAST
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-
C
Poincaré recurrence and maximum entropy
Fluctuation theorem says that there will be fluctuations in microscopic scale that results local decreases in entropy even in isolated systems. According to the Poincaré recurrence theorem, after sufficiently long time, any finite system can turn into a state which is very close to its initial... -
C
Blackbody Radiation - Entropy and Internal Energy
Homework Statement Expression for the entropy and internal energy of black body radiation. Using the below relations: Homework Equations Total free energy for black body: $$ F = (k_b TV/\pi^2) \int k^2 ln[1-exp(-\hbar ck/k_b T)]dk $$ Relationship between partition function and internal...- chris_avfc
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
P
Negative temperature and entropy
we know the entropy in each process increases is it true for absolute negative temperature?- persia7
- Thread
-
- Tags
- Entropy Negative Temperature
- Replies: 8
- Forum: Thermodynamics
-
D
Entropy of a continuous system
How could the entropy of a continuous system, like the electromagnetic field, be defined? Obviously you can't use something like the log of the phase space volume, but I can't think of anything that would work.- dEdt
- Thread
-
- Tags
- Continuous Entropy System
- Replies: 6
- Forum: Thermodynamics
-
T
Why is entropy defined as a state property?
Hi,I m just beginner in physics,but actually I don need exact expression of physical laws in math language.I know,math is language of physics but still... So,I m looking for answer to my questions about origin of Clausius s entropy. I this article: http://www.panspermia.org/seconlaw.htm is...- thedy
- Thread
- Replies: 1
- Forum: Thermodynamics