You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Mixing Definition and 298 Threads
In mathematics, mixing is an abstract concept originating from physics: the attempt to describe the irreversible thermodynamic process of mixing in the everyday world: mixing paint, mixing drinks, industrial mixing, etc.
The concept appears in ergodic theory—the study of stochastic processes and measure-preserving dynamical systems. Several different definitions for mixing exist, including strong mixing, weak mixing and topological mixing, with the last not requiring a measure to be defined. Some of the different definitions of mixing can be arranged in a hierarchical order; thus, strong mixing implies weak mixing. Furthermore, weak mixing (and thus also strong mixing) implies ergodicity: that is, every system that is weakly mixing is also ergodic (and so one says that mixing is a "stronger" notion than ergodicity).
View More On Wikipedia.org
The concept appears in ergodic theory—the study of stochastic processes and measure-preserving dynamical systems. Several different definitions for mixing exist, including strong mixing, weak mixing and topological mixing, with the last not requiring a measure to be defined. Some of the different definitions of mixing can be arranged in a hierarchical order; thus, strong mixing implies weak mixing. Furthermore, weak mixing (and thus also strong mixing) implies ergodicity: that is, every system that is weakly mixing is also ergodic (and so one says that mixing is a "stronger" notion than ergodicity).
View More On Wikipedia.org
-
S
MHB Mixing Teas for Profit: A 25% Return
A tea importer mixes tea bought at £5.20/kg with tea at £5.60/kg. He sells this blend at £6.80/kg making a profit of 25% on his cost price. In what ratio does he mix the teas?- splodge1
- Thread
-
- Tags
- Mixing
- Replies: 2
- Forum: General Math
-
C
Calculating Shaft Natural Frequency in Free Air and Liquid
Hello Fellow members. In many litterature about mixing liquids in tanks, they describe how to calculate the natural frequency of shafts in free air. But i have only found one book that describe about natural frequency of shafts in liquid, and it should have a damping effect of the natural...- Christoffer28
- Thread
- Replies: 2
- Forum: Mechanical Engineering
-
I
Temperature and entropy for two gases mixing
Homework Statement A system is made up of two halves. In one there's 10kg neon gas with the temperature ##20 \circ##C, in the other 10kg nitrogen gas with the temperature ##100 \circ## C. Suppose the septum is removed so that thermodynamic equilibrium may appear and the gases mix. Calculate...- Incand
- Thread
-
- Tags
- Entropy Gases Mixing Temperature
- Replies: 2
- Forum: Introductory Physics Homework Help
-
A
Risks of explosion mixing O2 and H2 in Ar magnetron plasma?
I am interested in adding simultaneously O2 and H2 in a magnetron sputtering discharge ignited in Ar at a pressure of 3*10-2 mbar. The discharge is driven by a RF generator (13.56 MHz, 60 - 120W). The target is W. Can anyone told me if I can expect at any explsion to apear due to simultanoously... -

Modified Huron-Vidal (1) mixing rule.
I have a question about this mixing rule. Seeing that it allows activity coefficients be used, and seeing that this mixing rule is very, very similar to the Predictive Soave-Redlich-Kwong (PSRK) equation of state's mixing rule, and seeing that other VLE simulators' PSRK VLE graphs are very...- maistral
- Thread
-
- Tags
- Mixing
- Replies: 1
- Forum: Materials and Chemical Engineering
-
E
How does split step Fourier method help four wave mixing?
Just a question How does solving the nonlinear schrodinger equation using split step Fourier method makes us understand the four wave mixing process in optical fiber ? Any examples on how that happens Thank you -
D
Exploring Pressure Changes When Mixing Hot Air and Steam in a Sealed Cylinder
Hi, everyone! I have a question: if we quickly fill a cylinder with steam from one end and with hot air from another end, both at atmospheric pressure and equal volume, with hot air 4 times hotter than steam, and then immediately seal that cylinder -- will pressure change as steam and hot air... -
T
Entropy change when mixing two gases
Homework Statement 1.00mole of nitrogen (N2) gas and 1.00mole of argon (Ar) gas are in separate, equal-sized, insulated containers at the same temperature. The containers are then connected and the gases (assumed ideal) allowed to mix. A) What is the change in entropy of the system? B) What is...- toothpaste666
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
E
Can someone explain how to setup this differential mixing problem
Homework Statement Setup a differential and solve the differential equation using Mathematica: Suppose water is added to a tank at 10 gal/min, but leaks out at the rate of 1/5 gal/min for each gallon in the tank. What is the smallest capacity the tank can have if the process is to continue...- erica
- Thread
-
- Tags
- Differential Explain Mixing
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-
E
Why large bandwidth is needed in Four Wave Mixing process?
Good Morning I loved the idea of four wave mixing in an optical fiber and how by satisfying phase matching condition you get a broad bandwidth but I want to know why am I interested in a large bandwidth why do I want to think about even enlarge it more? Thank you -

Why Does Heat Generate When Mixing Ethanol and Water?
Why heat is generated when ethanol is mixed with water? Please also explain why the existing hydrogen bond of water disintegrated to accommodate ethanol molecule?- nirmaljoshi
- Thread
- Replies: 6
- Forum: Mechanics
-

[itex]K^{0}-\bar{K}^{0}[/itex] mixing SQCD
I am trying to understand what the author means by the attachment (the underlined phrase). In my understanding if the masses were equal I should have: \frac{1}{(p^{2}-m^{2}+i \epsilon)^{2}} \sum_{ij} U^{d_{L}}_{di} U^{d_{L} \dagger}_{is}U^{d_{L}}_{dj} U^{d_{L} \dagger}_{js} why is the...- ChrisVer
- Thread
-
- Tags
- Mixing
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-

Why would mixing decrease the enthalpy?
Hello I am curious, why is the heat if mixing negative? Why would mixing decrease the enthalpy?- gfd43tg
- Thread
- Replies: 1
- Forum: Materials and Chemical Engineering
-

Chemistry redox reaction - gases mixing
Homework Statement How many milliliters of Cl2 gas, measured at 28.0 °C and 750 torr, are needed to react with 18.5 mL of 0.173 M NaI if the I- is oxidized to IO3- and the Cl2 is reduced to Cl-? Homework Equations The Attempt at a Solution So the molar mass is .0030906 mol NaI...- Feodalherren
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
I
Thermodynamics: Mixing ice and water
10 g of ice at -20C and 100g of water at +5C is mixed together. How much water and ice will there be once the system reaches equilibrium (assuming no heat is lost)? Specific heat of water and ice: 4.186*10^3, 2.108*10^3 Latent heat of fusion of ice: 333.55*10^3 All in units (kg^{-1} *...- iAlexN
- Thread
-
- Tags
- Ice Mixing Thermodynamics Water
- Replies: 4
- Forum: Introductory Physics Homework Help
-
A
MHB A question on ergodic theory: topological mixing and invariant measures
Hi All, This is a question on ergodic theory - not quite analysis, but as close as you can get to it, so I decided to post it here. Suppose I have a compact metric space $X$, with $([0,1], B, \mu)$ a probability space, with $B$ a (Borel) sigma algebra, and $\mu$ the probability measure...- Alex V
- Thread
-
- Tags
- Invariant Mixing Theory Topological
- Replies: 1
- Forum: Topology and Analysis
-
E
Four Wave mixing and split step Fourier method
Hello everyone . I need to ask if I want to get the right units for each parameter to be able to get the right results Can anyone define them properly? Like the gamma the initial power of pump or signal dispersion and non linear factors Thank you -

Is Work Meant to Be Positive and Heat Negative During Ethanol-Water Mixing?
Homework Statement Not a homework question exactly, but for a lab report I've looked at the changes in temperature and volume mixing different ratios (with same total mass) of ethanol and water. From this in the report I've been analysing questions such as "whether the work due to the volume...- AXidenT
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-

How Do You Relate Entropies to Temperatures in Water Mixing?
I don't know how to relate the entropies, S1 and S2, to the temperatures in my entropy balance -
C
Mixing Problem with Constant Coefficient Differential Equation
Homework Statement A tank contains 1160 L of pure water. A solution that contains 0.03 kg of sugar per liter enters a tank at the rate 7 L/min The solution is mixed and drains from the tank at the same rate. a.) How much sugar is in the tank initially? b.) Find the amount of sugar in the...- CollegeStudent
- Thread
-
- Tags
- Mixing
- Replies: 3
- Forum: Calculus and Beyond Homework Help
-

MHB ZZZZZzzz's question at Yahoo Answers regarding a mixing problem
Here is the question: I have posted a link there to this thread so the OP can find my work.- MarkFL
- Thread
-
- Tags
- Mixing
- Replies: 1
- Forum: General Math
-
A
The Mixing Problem: Understanding the Dilution of Brine in a Tank
Hi all. I'm trying to help a friend work a differential equation problem since I've already taken this course and gotten an A in it, but this other problem has me a little confused. The book is ODE's by Tenenbaum and Pollard, chapter 3 lesson 15a number 2. #1) Tank initially holds 100...- ank91901
- Thread
-
- Tags
- Mixing
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-
S
Real microstates change within mixing two gases
Hello, I'm looking for some changes of energy states of particles, as two gases are mixing together without total volume changing. That would explain increasing entropy better than their position combination. I know the particle energy states are getting more closely (the band gaps are reducing)...- sludger13
- Thread
-
- Tags
- Change Gases Microstates Mixing
- Replies: 1
- Forum: Atomic and Condensed Matter
-
C
Understanding K Mixing Level, K Selectivity and K Quantum Numbers
Can someone explain in simple terms "K mixing level" and "K selectivity" in nuclear decay processes ? ...as it relates to the "K quantum number". And does it relate directly to the nuclear angular momentum?...and selection rules? How so? Dumb it down for me please. Thank you kindly.- Creator
- Thread
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-
E
Mixing Gases, temperature, and the Kinetic Model of Matter
Homework Statement Consider the following statement: "The temperature of a gas is a measure of the speed of its particles (atoms and molecules). Now suppose that I mix two gases together, for example oxygen and carbon dioxide, which are initially at different temperatures; if I wait long...- evaengineer
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

MHB Alex's question at Yahoo Answers regarding a mixing problem
Here is the question: I have posted a link there to this topic so the OP can see my work.- MarkFL
- Thread
-
- Tags
- Mixing
- Replies: 1
- Forum: General Math
-
T
Archived What is the entropy of mixing for a system of two monatomic ideal gases?
I think I actually have solved it. I was right with the PV=nkT, I believe I previously messed up with the algebra. Homework Statement Using the same meathod as in the text, calculate the entropy of mixing for a system of two monatomic ideal gases, A and B, whose relative proportion is...- Thadis
- Thread
-
- Tags
- Calculation Entropy Mixing
- Replies: 1
- Forum: Introductory Physics Homework Help
-
K
Does mixing glycerol into water effect electrolysis of water?
Does mixing glycerol into water effect electrolysis of water? I tried measuring ohm and the resistance of water is lower after mixing glycerol. Then I experiment electrolysis of water, first I measure the resistance of DI water and it turns out that the resistant is about 0.63 mega ohm...- kevin_tee
- Thread
-
- Tags
- Electrolysis Mixing Water
- Replies: 14
- Forum: Chemistry
-
C
Exploring the Physical Phenomenon of Color Mixing
Why when artists mix blue and yellow colors, we see green? What is the physical explanation of this?- Chemist@
- Thread
-
- Tags
- Color Mixing Phenomenon Physical
- Replies: 6
- Forum: Other Physics Topics
-
F
Valence Band Mixing: What Is It & What Are References?
What does "valence band mixing" means? Could you provide me some reference about it?- fizikbrat
- Thread
-
- Tags
- Band Mixing Valence band
- Replies: 2
- Forum: Other Physics Topics
-
L
Simple D.E mixing tank problem
Hello, Having trouble with one quirk to this mixing tank problem: All large mixing tank initially holds 300 gallons of water, with 50 lbs of salt already in it (initial value problem). A brine solution of 2 lb/gal is pumped in at 3 gallons per minute. The water is pumped out at the faster...- leehufford
- Thread
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
L
MHB Mixing Teas: Solve to Find the Amounts of Each Kind!”
In a tea shop one mixes two different kinds of tea to get a new flavor. One of the kinds costs 135kr/kg, the other costs 168kr/kg. How large amounts of each kind have you taken if the mixture costs 150kr/kg? I tried setting up this equation system: 135x + 168y = 150 x+y=1 but I don't think it...- linapril
- Thread
-
- Tags
- Mixing
- Replies: 1
- Forum: General Math
-
H
MHB Calculation when Mixing Two Compounds....
Dear Comunity, I am running into a (probably pretty simple) problem here, yet the most simple things are sometimes upon me...:( I have a liquid solution which consists of 70% compound A and 30% compound B. I also have a bottle with 100% compound A. What I would like to do is to achieve a...- haubenkoch
- Thread
-
- Tags
- Calculation Compounds Mixing
- Replies: 2
- Forum: General Math
-
1
Calculus 2- Differential Equation Mixing Problem
Homework Statement A tank containing 400 liters of water has 10 kg of salt solute (dissolved salt). Some brine containing 0.03kg/L of salt is then introduced at a rate of 2 L/min. The solution is constantly mixed and evacuated at a rate of 2L/min, such that the volume remains constant. If...- 1LastTry
- Thread
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-
R
A Simple Salt Solution Mixing Problem - yet stuck
Hi, here is a very simple solution mixing problem that I can't solve which I am really ashamed of. Problem. A vessel whose capacity is 5 liters contains 2 liters of 15% salt solution. How many liters of 20% salt solution have to be mixed to the 15% solution to produce a solution with as high...- Rokas_P
- Thread
- Replies: 5
- Forum: General Math
-
R
Is additive color mixing a linear process?
Is additive color mixing a linear process (or close to)? (if this question has already been answered in detail, please forward me) If the answer to this question is "yes" then: Considering three hypothetical LEDs: Red T-1 (650nm λ), 60° viewing angle, 1000mcd luminous intensity... -
M
Maximizing Mixing Efficiency: Inline Mixing Pressures & PD Pump Selection
I have two streams at controlled flow rates that will mix via an inline static mixer. Both streams will have check valves before the mixer, however, do I have to worry about fighting pressures in this situation? If the one stream is 40 psi and the other is only 20 psi, will the 20 psi line... -
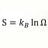
Entropy Of Mixing and Specific Heat
Homework Statement 100g of lead, specific heat .0345 cal/g/C at 100 C is mixed with 200 g of water at 20 C. Find the difference in entropy of system at end from value before mixing. Note: I think the book has wrong answer of 1.42 cal/K Homework Equations Before mixing entropy for water =...- morrobay
- Thread
- Replies: 17
- Forum: Introductory Physics Homework Help
-
S
Why is the mixing of ethanol and water exothermic?
Why is the mixing of ethanol and water exothermic? Because unless there's a reaction I'm thinking by the law of conservation of energy the total energy of the bonds should remain the same before and after. Because considering hydrogen bonds, the total number of bonds is dependent on the...- sgstudent
- Thread
-
- Tags
- Exothermic Liquids Mixing
- Replies: 9
- Forum: Chemistry
-
M
Spectra: line mixing explanation?
Hi Can anyone point me to a clear description of line mixing/"cross-relaxation" in atomic/molecular spectra? Every paper I read assumes some previous knowledge, is there an online resource that will explain why two transition would mix? I'm specifically looking for conceptual explanation...- mikeph
- Thread
-
- Tags
- Explanation Line Mixing Spectra
- Replies: 3
- Forum: Atomic and Condensed Matter
-
M
Archived Mixing steam at different pressures
Homework Statement steam flow through a line is 3.5 kg/s at 650 kps and .9 dry. It enters a mixing line where it exits at 800kpa, dry and saturated. The mixing steam is throttle from 2000kps to 800kpa. What is the mass of throttling steam added to have a final mixture of 800 kpa dry steam...- Morey
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
E
Products formed by mixing Sodium chloride and Potassium iodide?
I've looked at this website so many times and have been saved by it! Now I need a lil help please! I can finish all the calculations and balance it myself if I can just get the equation! Homework Statement Sodium chloride is placed in a solution of Potassium iodide. What products are...- edenlivori
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
M
How Do Box Diagrams Affect B-Bbar Mixing in the Standard Model?
Hi all, I'm being a bit thick in following some introductory lectures on the Standard Model: http://hepwww.rl.ac.uk/hepsummerschool/Teubner-%20Standard%20Model%202008.pdf The thing I'm struggling to understand is the treatment of how "box" diagrams contribute to B-Bbar mixing (see page 70...- muppet
- Thread
-
- Tags
- ckm ckm matrix Matrix Mixing
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
G
Mixing different pressures of air using valves
Hey guys! I've always been curious what would happen if I used air valves and regulators to mix different pressures of air together? For reference I spliced a few diagrams into one here: And I made a picture of the setup I'm imagining with some nice labels so we all know what...- Guilty Spark
- Thread
- Replies: 23
- Forum: Mechanics
-
K
Understanding Heat Transfer in a Ice-Water System
Homework Statement An 18g ice cube at -4.0ºC is placed into 75g of water at 10 ºC in an insulated container. a. What is the final temperature of the system? b. How much of the ice melts? I have a sheet full of these & ice to water to steam I really need somone to explain how to find the...- KylieB
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
S
Mixing of fluid streams in rivers
Walking round a local amenity area today brought this photo of a side stream cut off by the local river in spate flowing past from left to right in the picture. The river water is reddy brown and the side stream grey-green. The two waters are not mixing as can be seen by the well defined...- Studiot
- Thread
- Replies: 7
- Forum: Earth Sciences
-
A
Neutrino Mixing Angles: Explained & Mass Relation
Can someone please explain what neutrino mixing angles are and how they relate to neutrino mass.- AaronKnight
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
Mixing water at different temperatures
Hello all, I'm having trouble solving the given problem. "Assuming that no heat is lost to the surrroundings, what will be the final temperature when 1 kg of water at 10°C is mixed with 5 kg of water at 80°C" Taking the mean of the 2 fluids will not help here since their mass is...- MoniMini
- Thread
- Replies: 4
- Forum: Thermodynamics
-
Z
Diodes used for mixing - why needed?
Diodes used for "mixing" - why needed? At the site http://www.st-andrews.ac.uk/~www_pa/...rt1/page1.html there is a very comprehensive explanation of a "mixer diode". My question: if the diode is always forward biassed to produce an output frequency of fs - fl (source minus local frequency)...- zincshow
- Thread
- Replies: 6
- Forum: Electrical Engineering