- #36
- 23,594
- 5,836
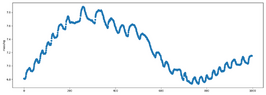
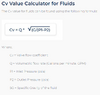
Is this what you are measuring inside the tank?Leopold89 said:No. What I mean is that I could for example measure a temperature of 25°C with a pressure of 10bar, then 26°C with a pressure of 8bar, 24°C with 4bar, 25°C with 2bar and so on. So what I mean is that my measurements may not be close to the line I calculated in post #32.