- #71
Leopold89
- 59
- 5
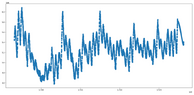
I tried something new and corrected the measured pressure of the CO##_2## tank with the atmospheric pressure. I expected to see a saw like pattern and hopefully small peaks dissapear, but got the plots attached here. The x axis is the Unix epoch time and the y axis is the difference between tank pressure and atmospheric pressure. Unfortunately the atmospheric pressure sensor is some 300 meters apart from the tanks.
I am rather discouraged, because we see a rising pressure over a few days and over three months the pressure difference never gets to 0, where I expect a change of tanks to have happened.
Maybe the whole experimental setup is faulty and needs revisiting?
I am rather discouraged, because we see a rising pressure over a few days and over three months the pressure difference never gets to 0, where I expect a change of tanks to have happened.
Maybe the whole experimental setup is faulty and needs revisiting?