shivajikobardan
- 637
- 54
- Homework Statement
- Difference between pyrolysis and gasification
- Relevant Equations
- none
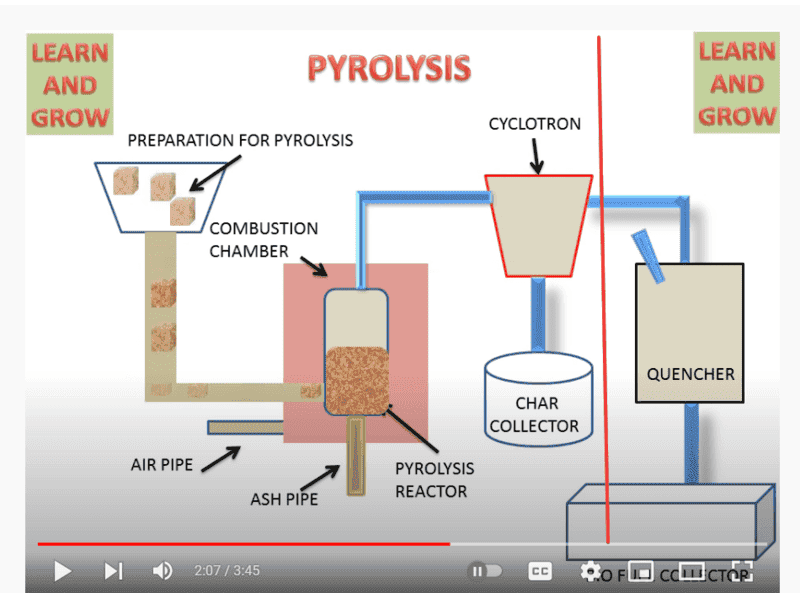
Pyrolysis-:
Gasification-:
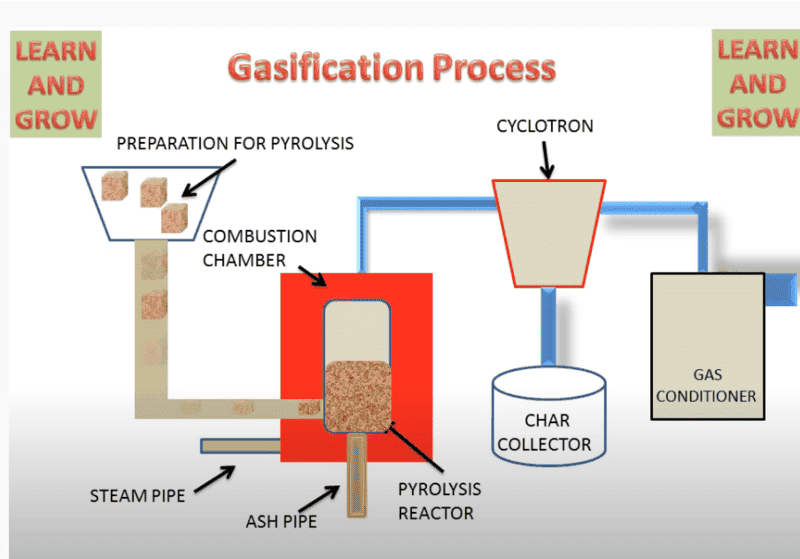
what's the difference between these two? i have in my notes that gasification uses partial air but pyrolysis uses no air. but in this figure gasification uses steam pipe...whereas pyrolysis uses air pipe. I can't understand what is going on.
Gasification-:
what's the difference between these two? i have in my notes that gasification uses partial air but pyrolysis uses no air. but in this figure gasification uses steam pipe...whereas pyrolysis uses air pipe. I can't understand what is going on.