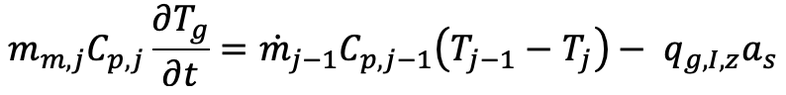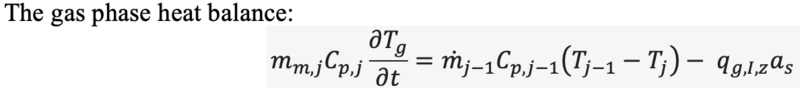casualguitar
- 503
- 26
Ok then similar to the Tuinier et al model you're saying that mass transfer rate will be proportional to the difference between the partial pressure of the species and the equilibrium vapour pressure of the speciesChestermiller said:Mass Transfer Rate for Deposition of CO2 and Water
The following is still somewhat approximate, but is a significant improvement to the mass transfer rate approach used by Tuinier et al:
$$\dot{M}_i^"=k_i\left(\frac{Py_i-p_i(T_I)}{RT_I}\right)\tag{1}$$where ##\dot{M}_i^"## is the molar flux of species per unit area of surface, P is the total pressure, ##y_i## is the mole fraction of species in the bulk gas, ##p_i(T_I)## is the equilibrium vapor pressure of species at the interface temperature ##T_I##, and ##k_i## is the mass transfer coefficient (units of m/s) of species for deposition from the gas.
Where does equation 1 come from? Is this an equation you derived or is it an established equation?
Got it, so I guess we will be able to find Sherwood number correlations for packed beds in Bird et al that we can use here to solve for the mass transfer coefficientChestermiller said:The mass transfer coefficient ki is related to the local Sherwood number for mass transfer by Shloc,i=kiDp(1−ϵg)Diψwhere Di is the diffusion coefficient of species in N2 and ψ is the packing particle shape factor (equal to 1.0 for spherical particles and 0.92 for cylindrical particles).
So if the Nusselts number tells you how convection or conduction dominant the heat transfer is, the Sherwood number tells you how convection or diffusion dominant the mass transfer is?Chestermiller said:By Reynolds analogy, the Sherwood number Shloc,i is the same function of Reynold number and Schmidt number as the Nussult number is as a function of Reynolds number and Prantdl number, respectively.
Got itChestermiller said:An additional constraint on Eqn. 1 is that M˙i" must be zero if the number of moles of deposit per unit area of surface is zero and the right hand side of Eqn. 1 is negative. This means that the number of moles per unit area of deposit on the surface of the particles can never go negative.
This just leaves us with the ##U_g## and ##U_b## correlations then? On this now
Last edited: